The test is a real-time RT-PCR amplification and detection system that utilizes a bifunctional fluorescent probe-primer for the detection and differentiation of human influenza A virus RNA human influenza B virus RNA and RSV RNA in nasopharyngeal swabs NPS. Real-time polymerase chain reaction PCR laboratory developed test LDT.
Opti Sars Cov 2 Influenza A B Rt Pcr Test Opti Medical Systems
If repeated positive results for multiple HA targets are obtained this raises the possibility of co-infection which should be confirmed by sequencing or virus culture.
. But also in the case of the Nagy v2 M-gene assay swine swine influenza virus SwIV human IAV and IAVs from other mammalian hosts. The RNA extraction control may be an H5H7 AIV isolate and provided that this has given satisfactory results in the H5 H7 and or M gene RRT-PCR then there is no need to include an N5 -positive extraction control. M Panning 1 M Eickmann 2 O Landt 3 M Monazahian 4 S Ölschläger 5 S Baumgarte 6 U Reischl 7 J J Wenzel 7 H H Niller 7 S Günther 5 B Hollmann 1 D Huzly 1 J F Drexler 8 A Helmer 8 S Becker 2 B Matz 8 A M Eis-Hübinger 8 C Drosten 8.
Common signs of an influenza infection include. A rapid low cost accurate point-of-care POC device to detect influenza virus is needed for effective treatment and control of both seasonal and pandemic strains. Real-time RT-PCR of in vitro-transcribed H3N2 RNA revealed a standard curve for quantification with a linear range correlation coefficient 0.
N5 AIV RNA dilution series standards. After a detection range was established we tested serial 5-fold dilutions of extracted RNA from each virus at titers near the limit of detection LOD with the Flu SC2 Multiplex Assay the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel or the CDC rRT-PCR Flu Dx Panel. The test is a real-time PCR amplification and detection system that utilizes a bi-functional fluorescent primer-probe for the detection of human influenza A virus RNA and the differential.
The CDC Influenza SARS-CoV-2 Flu SC2 Multiplex Assay is a real-time reverse-transcription polymerase chain reaction RT-PCR test that detects and differentiates RNA from SARS-CoV-2 influenza A virus and influenza B virus in upper or lower respiratory specimens. Influenza A symptoms. One -step conventional RT-PCR Influenza B lineage-specific.
Runny or stuffy nose. Influenza types A and B are the most common causes of flu in Australia and can cause major outbreaks and severe disease. Real-time polymerase chain reaction rt-pcr Reference ranges.
If you get it you will need to rest at home and avoid infecting others. You just use an influenza primer rather than one for SARS-CoV-2. Vaccination can protect you against influenza A.
Looking for influenza uses the same trick. Flu ABRSV Influenza virus AB RNA QL Real Time PCR. Use an N5 AIV RNA preparation from extracted egg fluid recommended.
The assay is composed of 2 principal steps. RT-PCR procedure using a new RNA extract from the original specimen or an RNA extract from another specimen. The assay provides a sensitive nucleic-acid-based diagnostic tool for evaluation of specimens.
Other acceptable specimens are a nasal andor throat swab. Unlike a common cold the flu typically occurs with a sudden onset of symptoms. Influenza AB Typing Kit version 2 510 k no.
This test was developed and its performance characteristics have been determined by. Detection of influenza A H1N1v virus by real-time RT-PCR. The best upper respiratory tract specimens to detect influenza viral RNA by RT-PCR and other molecular assays are nasopharyngeal swabs washes or aspirates.
AIV and SwIV RNA is extracted from clinical specimens IAV cultures amplified in avian usually chicken embryonated eggs and cell cultures avian and or mammalian. A swab with a wood shaft should not be used for respiratory specimen collection because it may interfere with RT-PCR and other molecular assays. The DNA primer added has a.
We developed a single-use microfluidic chip that integrates solid phase extraction SPE and molecular amplification via a reverse transcription polymerase chain reaction RT-PCR to amplify. 1 extraction of RNA from patient. Upon optimization of the assay conditions all the major influenza A virus subtypes including H1N1 H3N2 H5N1 H7N3 and H9N2 were amplifiable by this method and had a PCR product length of 179 bp.
RNA originating from avian avian influenza virus AIV. Influenza A is a type of virus that causes influenza the flu a highly contagious respiratory illness.
Realstar Influenza Rt Pcr Kit Altona Diagnostics
Biosensors Free Full Text Nucleic Acid Based Sensing Techniques For Diagnostics And Surveillance Of Influenza Html
Label Free Fluorometric Detection Of Influenza Viral Rna By Strand Displacement Coupled With Rolling Circle Amplification Analyst Rsc Publishing Doi 10 1039 D0an01326a
Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine
Label Free Fluorometric Detection Of Influenza Viral Rna By Strand Displacement Coupled With Rolling Circle Amplification Analyst Rsc Publishing Doi 10 1039 D0an01326a
Direct Viral Detection Via Qpcr
Biosensors Free Full Text Nucleic Acid Based Sensing Techniques For Diagnostics And Surveillance Of Influenza Html
Label Free Fluorometric Detection Of Influenza Viral Rna By Strand Displacement Coupled With Rolling Circle Amplification Analyst Rsc Publishing Doi 10 1039 D0an01326a
Detection Of Rna Viruses From Influenza And Hiv To Ebola And Sars Cov 2 A Review Analytical Methods Rsc Publishing Doi 10 1039 D0ay01886d
Comparison Of The Cepheid Xpert Xpress Flu Rsv Assay And Commercial Real Time Pcr For The Detection Of Influenza A And Influenza B In A Prospective Cohort From China International Journal Of Infectious
Microfluidic Chip For Molecular Amplification Of Influenza A Rna In Human Respiratory Specimens Plos One
Viruses Free Full Text Live Attenuated Influenza Vaccine Contains Substantial And Unexpected Amounts Of Defective Viral Genomic Rna Html
Rapid Combined Flu A B And Sars Cov 2 Rna Polymerase Chain Reaction Testing For Emergency And Ambulatory Care Settings
Protocol For Influenza A Virus Infection Of Mice And Viral Load Determination Star Protocols
Automated Multiplex Nucleic Acid Tests For Rapid Detection Of Sars Cov 2 Influenza A And B Infection With Direct Reverse Transcription Quantitative P Rsc Advances Rsc Publishing Doi 10 1039 D0ra04507a
Simultaneous Detection And Differentiation Of Sars Cov 2 Influenza A Virus And Influenza B Virus By One Step Quadruplex Real Time Rt Pcr In Patients With Clinical Manifestations International Journal Of Infectious Diseases
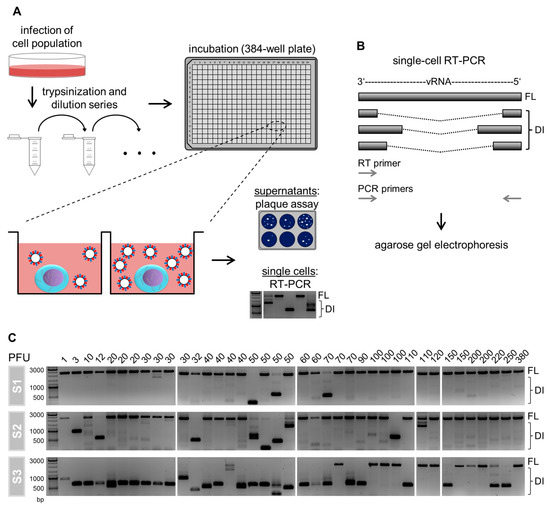
Viruses Free Full Text Single Cell Analysis Uncovers A Vast Diversity In Intracellular Viral Defective Interfering Rna Content Affecting The Large Cell To Cell Heterogeneity In Influenza A Virus Replication Html
- lukisan 3d gajah
- osaka castle nishinomaru garden
- gambar batu quran
- cuti sekolah johor 2019
- motor roda tiga samping
- karangan tentang sambutan hari raya di sekolah
- bursa malaysia securities berhad
- cara memasak mie kuning untuk bakso
- kata mutiara bangkit dari terpuruk
- minamas plantation sime darby group
- gambar lukisan bunga cantik
- lori pasir 10 tayar
- air zam zam tokopedia
- psychology course in malaysia
- undefined
- influenza a rna pcr
- cara membuat gambar burung elang
- mesir vs arab saudi
- arti nama zahra ramadani
- jus tomat bersifat
